Abstract
Introduction: Factors contributing to the antitumor activity of systemic oncolytic virotherapy (OV) include (i) generalized innate immune activation due to virus infusion; (ii) direct oncolytic and/or immune-mediated destruction of OV infected tumor cells due to intratumoral spread of the virus infection; (iii) collateral boosting of tumor antigen-specific T cell responses that target uninfected tumor cells. We investigated the relative contributions of these 3 factors T cell lymphoma (TCL) patients (pts) responding to a single intravenous infusion of Voyager-V1, an oncolytic vesicular stomatitis virus (VSV) encoding IFNβ and sodium iodide symporter (NIS) on NCT03017820. Of relevance, the virally encoded IFNβ enhances tumor specificity, boosts proinflammatory activity & serves as a convenient serum biomarker of infection.
Methods: In a first in human trial, a single infusion of Voyager-V1 was administered to 15 pts with relapsed refractory hematologic cancers; 4 dose levels (DL) were tested (5x10 9, 1.7x10 10, 5x10 10, 1.7x10 11 TCID50); 7 pts had TCL. Cytokine levels and CRS symptoms were determined as a measure of generalized immune activation; serum IFNβ levels were monitored as a pharmacodynamic biomarker of virus infection; antitumor T cell responses were measured using ELISPOT assays.
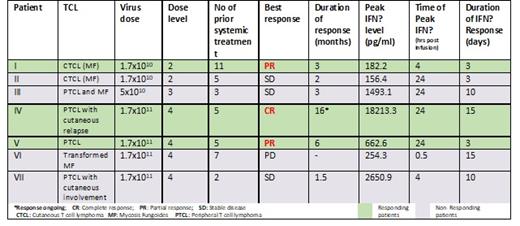
Results: Of 8 TCL pts, 3 had RECIST responses: PR at DL 2, a CR & PR at DL 4. Most pts experience short lived CRS typically resolving within 24 hours of virus infusion. Serum IFNβ levels increased post-therapy in all pts, typically peaking by 24 hours post virus infusion and returning to baseline by median day 10. T cell responses could not be accurately determined in most cases due to suboptimal T cell viability and/or function after thawing of frozen PBMC. Factors contributing to tumor regression in each of the responding pts appeared to differ significantly, as exemplified by the case reports below.
Pt (I) with CTCL experienced grade 1 CRS followed by brisk regression of multiple cutaneous lesions. The PR lasted 3 mo, after relapse of an eye lesion, but responses at other sites deepened after the relapsed lesion was irradiated; pt remained off active therapy for the next 2 yrs. Peak serum IFNβ 4 hours post infusion was only 182 pg/ml suggesting that disease response may have been driven by nonspecific innate immune activation associated with the virus infusion.
Pt (IV) (CD30+ PTCL/ cutaneous relapse) had short lived grade 2 CRS, then developed signs of IFNβ toxicity with AST elevation and thrombocytopenia. Oral ruxolitinib was administered for 5 days as IFNβ toxicity mitigation leading to normalization of AST and platelets. All PET-avid tumors regressed completely. This CR continues 16 months post virus infusion. Peak serum IFNβ was 18,213 pg/ml strongly suggesting that response was driven by extensive intratumoral amplification of the virus and oncolytic tumor destruction. Robust CTL responses to tumor neoantigens were detected in this pt at baseline indicating that collateral boosting of tumor-specific T cells may have been responsible for the long term maintenance of this CR
Pt (V) (CD30+ PTCL) had short lived grade 2 CRS followed by response at all disease sites (including complete normalization of an enlarged FDG-avid spleen). Her PR ended after 6 months due to relapse in cervical lymph nodes. Peak serum IFNβ level was modest, 600 pg/ml & sustained over 48 hours suggesting slower intratumoral spread of the virus infection. The deepening of the response long after resolution of the oncolytic phase points strongly to a contribution from antitumor CTL but the assay was technically unsatisfactory in this pt.
Conclusion: Analysis of the correlative data supports that intravenous Voyager-V1 therapy can recruit diverse antitumor effector mechanisms to impact tumor response in pts with heavily pretreated TCL. Importantly, intratumoral virus amplification, which results in high circulating concentrations of IFNβ, does not necessarily lead to tumor regression perhaps because of defective immune cell recruitment and/or activation in some tumors but not in others. This will be further evaluated in future study participants by performing CTL assays on fresh, rather than frozen PBMC. In conclusion, a single IV infusion of Voyager-V1 shows highly promising activity against treatment refractory TCL and should be further advanced as a new single-shot or combination therapy for this therapeutically challenging indication.
Witzig: Karyopharm Therapeutics, Celgene/BMS, Incyte, Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS, Acerta Pharma, Kura Oncology, Acrotech Biopharma, Karyopharm Therapeutics: Research Funding. Paludo: Karyopharm: Research Funding. Patnaik: StemLine: Research Funding; Kura Oncology: Research Funding. Dispenzieri: Takeda: Research Funding; Alnylam: Research Funding; Pfizer: Research Funding; Oncopeptides: Consultancy; Sorrento Therapeutics: Consultancy; Janssen: Consultancy, Research Funding. Bennani: Kymera: Other: Advisory Board; Vividion: Other: Advisory Board; Kyowa Kirin: Other: Advisory Board; Daichii Sankyo Inc: Other: Advisory Board; Purdue Pharma: Other: Advisory Board; Verastem: Other: Advisory Board. Ansell: Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium and Takeda: Research Funding. Gertz: Aurora Biopharma: Other: Stock option; Akcea Therapeutics, Ambry Genetics, Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Karyopharm Therapeutics, Pfizer Inc (to Institution), Sanofi Genzyme: Honoraria; Akcea Therapeutics, Alnylam Pharmaceuticals Inc, Prothena: Consultancy; AbbVie Inc, Celgene Corporation: Other: Data Safetly & Monitoring; Ionis Pharmaceuticals: Other: Advisory Board. Dingli: Janssen: Consultancy; Sanofi: Consultancy; GSK: Consultancy; Apellis: Consultancy; Novartis: Research Funding; Alexion: Consultancy. Kumar: Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Consultancy; Carsgen: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Merck: Research Funding; Oncopeptides: Consultancy; Roche-Genentech: Consultancy, Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy, Honoraria; Tenebio: Research Funding; Novartis: Research Funding; Bluebird Bio: Consultancy; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding. Bergsagel: Oncopeptides: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Genetech: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Patents & Royalties: human CRBN mouse; Janssen: Consultancy, Honoraria. Kapoor: Karyopharm: Consultancy; Cellectar: Consultancy; BeiGene: Consultancy; Pharmacyclics: Consultancy; Sanofi: Consultancy; Amgen: Research Funding; Ichnos Sciences: Research Funding; Regeneron Pharmaceuticals: Research Funding; Glaxo SmithKline: Research Funding; Karyopharm: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; AbbVie: Research Funding. Lin: Sorrento: Consultancy; Vineti: Consultancy; Legend: Consultancy; Novartis: Consultancy; Bluebird Bio: Consultancy, Research Funding; Merck: Research Funding; Juno: Consultancy; Celgene: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Gamida Cell: Consultancy; Janssen: Consultancy, Research Funding; Takeda: Research Funding.
VSV-IFNβ-NIS is an oncolytic virus in clinical trial for systemic administration in hematologic malignancies

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal